Does Galvanized Steel Rust
Galvanized steel has been used for almost 2,000 years because of its unrivaled ability to last a very long time and resist rust. Hot dipped galvanized steel and electroplated galvanized steel are made using different methods and their zinc galvanized coatings corrode completely differently. Learn about these galvanizing processes (here) and how zinc corrosion varies between them (here).
Yes, galvanized steel resistance to rust corrosion depends largely on the type and thickness of the protective galvanized zinc coating, but the type of corrosive environment is also a critical factor.
Factors that rust and corrode galvanized steel:
- Relative humidity above 60%
- Sodium chloride (salt) in water or air
- Wet or soaked environments
- Increase in temperature when combined with corrosive factors like humidity and industrial pollution
- Acids; particularly sulfur acids produced by (1) hydrogen sulfide - from volcanoes, hot springs, natural gas, and sewer gas - and (2) sulfur dioxide pollution in the urban atmosphere
- Strong Alkalis
- Plasters and cements (especially Portland cements) containing chlorides and sulfates
- Acid rainwater runoff from roofs with wood shingles
- Moss and lichen
- Contact between galvanized items and copper, pure iron, or steel causes galvanic corrosion. Galvanic (electrochemical) corrosion is an electrolytic corrosion reaction between the zinc coating and dissimilar metals when in the presence of an electrolyte such as rain, dew, fog or condensation.
- Acidic food and drinks (is galvanized metal safe for food?)
Galvanized steel has good resistance to:
- Concrete
- Mortar
- Lead
- Tin
- Zinc
- Aluminum
Galvanized steel is corrosive to all metals except lead, tin, zinc and aluminum.
Although it does not last infinitely, galvanized steel is the unparalleled corrosion-resistant metal. It is worth noting however, that applying a protective coating such as paint to galvanized steel will alleviate the problems caused by corrosion of the protective zinc coating.
How Long Does Galvanized Steel Last
So how long does it take for a handy new galvanized steel bucket to rust and corrode into a useless heap of metal? It takes a long time. A galvanized steel bucket (produced with any method) can last practically forever if it's gently used and kept dry and out of the rain. But for those galvanized buckets and tubs destined to become garden planters, landscape decorations, animal feeders, and farm water buckets corrosion is inevitable. Galvanized steel intended for prolonged outdoor use should be hot-dipped galvanized steel; which commonly lasts for about 70 years in many different environments.
Table 1 below predicts how long galvanized steel will last based on a 30 month corrosion study of environmental factors like wetness, humidity, and air pollutants in 2004.
| Table 1. Prediction of When Zinc Layer will be Consumed on Galvanized Steel | |
|---|---|
| Galvanized Steel kept in the wet or soaked environments | 10 Years |
| with a relative humidity of 100% | 34 Years |
| with a relative humidity below 60%. | 211 Years |
Source: Journal of Materials in Civil Engineering 2004 (11)
The corrosion resistance of zinc coatings is determined primarily by the type and thickness of the coating but, varies with the severity of environmental conditions exposed to (as in the table above). Hot dipped galvanized zinc coating resistance to corrosion depends primarily on a protective film (patina) formed on its surface.
Read more background:
- Types of galvanizing; compare the properties of galvanization methods.
- Does galvanized zinc rust; learn how a hot-dipped zinc coating corrodes to form a patina layer that can protect the zinc metal underneath for upwards of 75 years. Zinc corrosion products of hot-dip galvanizing build-up (creating a patina layer) and insolubly cling to the metal in many environments. Thus, the corrosion rate of hot-dip galvanized steel may slow as time progresses.
The type of zinc galvanization and how that process controls the way in which the galvanized steel corrodes must be understood first. The environments, elements, and conditions that any given type of galvanized steel is exposed to, nevertheless, indeed determines how long it will last before corrosion.
A 1926 study of galvanized steel corrosion in industrial, rural and sea regions found:
- At any one location the life of the zinc coating is directly proportional to its thickness.
- The most rapid corrosion occurred at the highly industrial locations, and the least rapid at the rural and more arid locations (9).
The handy chart below (from American Galvanizers Association) illustrates how long galvanized steel will last before corroded areas should be maintained to prevent further deterioration. Want to learn how to refinish galvanized steel? Click here. Put another way, this chart shows how long it takes for galvanized steel to rust in different environments.
The thicker the zinc coating the longer galvanized steel will last without corrosion. The thickness of zinc is displayed along the horizontal axis (8). As in the chart below and noted in the 1926 study, for each location the corrosion rate is essentially constant with time (9).
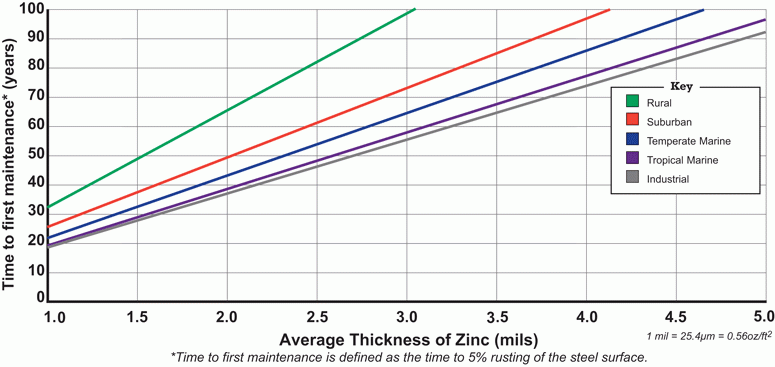
Chart 1: Time to first sign of corrosion in various environments
The environments below are listed from the most corrosive to the least corrosive:
Industrial Environments:
- Most city and urban areas as examples of urban environments.
- Generally, the most aggressive corrosive environment.
- Sulfide and phosphate air pollution, from point sources like automobile exhaust, cause galvanized zinc coating consumption.
Tropical Marine Environments
- Regions where the temperature, if ever, falls below freezing.
- Humidity is high and chlorides from nearby water are present in air.
- Almost as corrosive as industrial environments
- Warm temperatures increase the activity level of corrosion elements on the surface of the galvanized zinc.
- Proximity to the coast, wind direction and wind speed also influence the rate of corrosion
Temperate Marine Environments
- Lower temperatures and humidity make temperate marine environments less corrosive to their tropical counterparts.
- Like tropical marine regions, chlorides, distance from the ocean, wind direction, and wind speed shape corrosion rate.
Suburban environments
- Less corrosive than industrial areas
- Residential perimeter communities outside urban areas and cities.
Rural Environments
- Least aggressive corrosive atmosphere
- Air and rain in rural regions contain relatively low levels of sulfur and other corrosive emissions.
Elements and conditions:
Air
Sulphur Dioxide (SO2) is the most significant atmospheric air pollutant. The presence of SO2 in the atmosphere largely regulates the atmospheric corrosion rate of zinc. When acids - with a pH below seven - attack and corrode a galvanized zinc coating, the pH decreases and the rate of corrosion increases. In industrial locations mists and dews have been observed having a pH as low as three. It is rational, therefore, to attribute the greater corrosivity of industrial atmospheres to the acid-forming SO2 pollution contained within them (9).
Results of a galvanized zinc metal corrosion potential study published in 2015 found the highest corrosion impact from SO2, dust, humidity and CO2. Concentrations of these pollutants were highest values in winter; when fossil fuel combustion increases. The presence of chlorides in air during also influenced the rate of corrosion (10).
Soil
Just as the acidity of the atmosphere influences the rate of corrosion, so too does the acidity of the soil. The zinc coating of hot-dipped galvanized steel will last in the harshest soil is 35 to 50 years and in less corrosive soil 75 years or more.
Temperature
Although humidity affects corrosion, temperature itself has less of an impact. Galvanized zinc coatings respond well in extreme cold and hot temperatures. There are no significant differences in corrosion rate in temperatures below -40 F for hot-dip galvanized coatings. In higher temperatures the zinc can be impacted. For long-term continuous exposure the maximum recommended temperature is 392 F, according to a publication by American Galvanizers (8). Temperatures this high can cause the outer zinc layer to peel away from the zinc-alloy layers. Although the remaining zinc-alloy layers will provide corrosion protection to the steel, protection will last for less time than if the outer free zinc layer remained intact.
Because the applications of steel are many, hot-dip galvanizing will continue to be called upon to ensure long-lasting and maintenance-free corrosion protection.
What are the best storage conditions for galvanized buckets?
Although these buckets can take a fair about of abuse, for optimum performance choose a storage area that has adequate ventilation and has a low amount of moisture. Avoid areas that are damp and poorly ventilated.



