Does Zinc Rust
Yes, zinc of galvanized steel rusts, but not in the same way as other metals. Layers of zinc corrode differently depending on which type of galvanization method is used. For hot-dipped galvanizing, when a galvanized steel coating of zinc corrodes with the atmosphere or water a "patina" layer of corrosion byproducts is created. This thin outer layer of zinc rust is white, it protects the zinc metal underneath and it does not wash off. Long term corrosion protection of the zinc coating depends on the formation of the patina layer; or the exposed rusting of the zinc coating.
Earlier we discussed zinc plated steel and zinc coatings: learning how zinc protects the underlying metal from corrosion and how the type of galvanization affects the properties of the zinc metal alloys and rate of corrosion. Figure 1 below illustrates the zinc thickness of various galvanizations bonded to base steel.
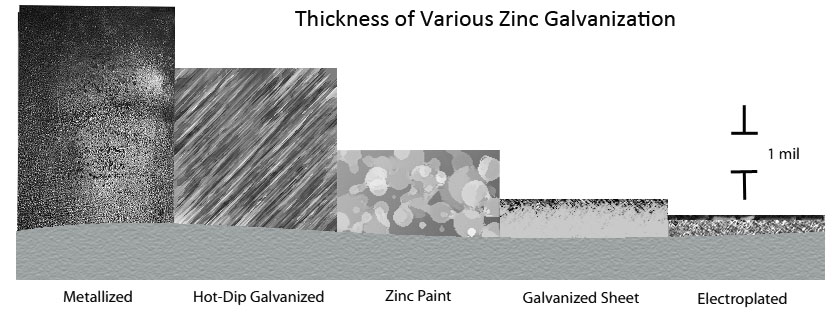
Figure 1: Thickness and microstructure of various zinc coatings (6 p.1)
Electroplate galvanization to apply a zinc layer has advantages, but zinc does not form a patina layer or white rust. If the outer layer of electroplated galvanized zinc is damaged, the underlying steel will be exposed to the environment and rust. Electroplate galvanized steel left outside for about a decade or two will weaken the zinc coating and eventually expose the underlying steel to corrosion and rust; far sooner than hot-dipped galvanized steel.
All zinc galvanized coatings are more corrosion resistant than bare iron or steel. Like all ferrous metals, zinc corrodes when exposed to air and water. However, zinc corrodes at a rate of 1/30 of that for steel. Also like other ferrous metals, zinc corrodes or rusts at different rates depending on its environment (8).
Common environments for galvanized steel are indoor and outdoor environments, storing hundreds of different chemicals, in fresh water, sea water, soils, concrete, treated wood, or extreme temperatures. The performance of galvanized steel depends on the environment as well as the galvanization process.
Related: How long does galvanized steel last?
Hot-dipped galvanizing creates a zinc barrier and sacrificial cathodic protection to prevent corrosion of the steel itself. The zinc is protected by the formation of a patina layer on the surface of the coating. The patina layer is the products of zinc corrosion and rust. The first products of zinc corrosion are zinc oxide and zinc hydroxide. Later these products react with atmospheric carbon dioxide to form zinc carbonate. The zinc carbonate is the stable film that adheres to the zinc surface and won't wash off. The zinc carbonate layer corrodes very slowly and protects the zinc underneath. The formation of zinc carbonate turns the zinc coating to a dull grey color.
Thus, for hot-dip galvanizing, the zinc carbonate layer is the rusted or corroded zinc that protects the zinc coating protecting the steel.



